Warning: This article contains strong language. If you don't like strong language, then leave this page immediately.
In Part 1, I shared the story of Adriano who, upon his doctor’s advice, began taking an antidepressant last year. Rather than help with his anxiety and depression, the SSRI drug prescribed to Adriano made his condition much, much worse. He became increasingly agitated, jittery, impulsive and aggressive.
Worst of all, Adriano became suicidal.
Shocked by what Adriano had experienced as a result of this so-called ‘antidepressant’, I promptly delved into the published research on these drugs. Was Adriano’s reaction atypical, or was he the victim of yet another Big Pharma scam?
The Great Serotonin Scam
The drug prescribed to Adriano was fluvoxamine, originally marketed as “Luvox”. It belongs to a class of drugs known as selective serotonin reuptake inhibitors (SSRIs), whose alleged ‘antidepressant’ mechanism is to inhibit binding of the neurotransmitter serotonin in the brain. This supposedly leaves more 'free' serotonin available at the all-important synaptic gap between brain neurons. This increase in available serotonin, so the story goes, relieves depression and anxiety because serotonin is a "happy hormone" that makes you calm and tranquil. The basic equation behind the marketing of SSRIs to the medical profession and public alike is that more serotonin = less sadness and more happiness.
The SSRI theory is simplistic to the point of stupidity. Seriously, I've seen more intelligent hypotheses in children's books.
First of all, no-one has ever demonstrated a cause-and-effect relationship between serotonin levels in the brain and depression, anxiety or any other mood disorder.
Secondly, even if it were to be established that boosting brain levels of serotonin improved mood, there's a little something known as "homeostasis" that would very likely ruin the party in short order. Homeostasis is the well-known tendency of the body to "revert to the mean". In other words, the human body tends to operate within a certain range of temperature, hormone levels, fluid concentrations, and so on. Substantial fluctuations from this relatively narrow range are quickly countered by the body.
For example, if you drink large amounts of water, in excess of your hydration needs, you will not start storing water like a camel. Your body will simply urinate more to get rid of the excess water.
Another example: If you take large amounts of anabolic steroids, for a long enough period of time, your own natural production of testosterone will greatly decline. If you suddenly stop taking the anabolics, your pharmaceutically bolstered levels of testosterone will quickly plummet and you will be left with very low testosterone levels. There will then be a transition period in which your body desperately scrambles to restore your own endogenous testosterone production back to former levels.
We've known about homeostasis for eons, and phenomena like the two examples above are hardly state secrets. Yet along comes Big Pharma, and somehow convinces the entire medical profession that taking SSRIs will boost serotonin levels indefinitely. Setting aside the fact that this may not necessarily be a good thing, few people have stopped to consider the body may counter the increase in serotonin by decreasing its own natural serotonin production, or by decreasing the brain’s sensitivity to serotonin.
A Pioneer Serotonin Researcher Debunks the “Chemical Imbalance” Theory
David D. Burns received his medical degree from Stanford University School of Medicine and completed his psychiatry residency at the University of Pennsylvania School of Medicine. After his residency, he completed three years of research as a post-doctoral fellow. During that time, he addressed the theory that depression and anxiety were the result of a "chemical imbalance" in the brain; specifically; that patients with depression had a serotonin deficiency, and patients with mania had an excess. Burns could not find any evidence that any psychiatric disorder resulted from a "chemical imbalance" in the brain.
Burns was given the prestigious A. E. Bennett Award for his research on brain serotonin metabolism. When asked about the scientific status of the serotonin theory in 2003, this is what he said:
“I spent the first several years of my career doing full-time research on brain serotonin metabolism, but I never saw any convincing evidence that any psychiatric disorder, including depression, results from a deficiency of brain serotonin. In fact, we cannot measure brain serotonin levels in living human beings so there is no way to test this theory. Some neuroscientists would question whether the theory is even viable, since the brain does not function in this way, as a hydraulic system.”
I’ll further dismantle the shambolic "chemical imbalance" theory in a future instalment. For now, let’s return our focus to SSRIs and specifically fluvoxamine.
SSRIs are unleashed on the World
Prior to the advent of SSRI drugs, the most widely-prescribed antidepressants were of the tricyclic variety. Because these drugs had a litany of side effects and were often rough on patients’ cardiovascular systems, researchers began searching for less toxic alternatives.
The medical world thought they’d stumbled upon this magical alternative with the advent of SSRIs. In 1982, the first SSRI - a drug known as zimelidine – was brought to market. It was withdrawn from sale the following year when it became apparent the drug caused liver toxicity, severe headaches, and greatly increased the risk of developing Guillain–Barré syndrome, a rather nasty autoimmune disorder. The risk of developing Guillain-Barré syndrome was increased around 25-fold among patients receiving zimeldine, compared with the natural incidence of the disorder.
Oops.
In 1983, a drug called indalpine became the second SSRI to appear on pharmacy shelves. Marketed as Upstene, indalpine’s days were also numbered. The drug was pulled from the market in 1985 after displaying a penchant for markedly reducing patients’ white blood cell counts. In its first year, 30 cases of neutropenia, agranulocytosis and leucopenia were reported in indalpine patients, five of which were fatal. In indalpine’s second and final year, 32 cases were reported.
The third SSRI to hit the market was fluvoxamine. Developed by Phillips-Duphar in the Netherlands, it was introduced as Floxyfral in Switzerland in 1983. In 1994, fluvoxamine became available in the US after the Food and Drug Administration (FDA) approved it for the treatment of obsessive-compulsive disorder.
In 1986, fluoxetine – more famously known as Prozac – first became available in Belgium. The following year the FDA approved its sale in the US as a treatment for depression. As such, Prozac was the first SSRI to be sold in the highly lucrative US market.
These drugs were joined by sertraline (Lustral, Zoloft), paroxetine (Seroxat, Paxil), citalopram (Cipramil, Celexa) and the most recent SSRI, escitalopram (Lexapro).
In this article, I’ll focus on fluvoxamine, as it was the drug given to Adriano, and therefore the drug that prompted me to begin investigating the shady world of antidepressants. Rest assured, in Part 3 I’ll put the other SSRIs under the microscope.
After Adriano quit fluvoxamine, my first port of call was PubMed, where I typed in the keywords “fluvoxamine and suicide.”
Within minutes, I retrieved studies showing this allegedly “remarkably safe” drug increases the odds that users will try to harm and kill themselves. Before I share these studies, let’s revisit an event that gave a chilling insight into the violent potential of this toxic agent.
Bowling for Fluvoxamine
At 11:19am on April 20, 1999, at Columbine High School in Columbine, Colorado, twelfth graders Eric Harris and Dylan Klebold began shooting at their fellow students. By the time their rampage was over, ten students and one teacher had been murdered. Twenty-four additional people were injured. After the shootings, Harris and Klebold committed suicide in the school library. At the time, it was the deadliest high school shooting in US history.
Both shooters were autopsied after the massacre. The toxicology analysis showed Harris’ blood was free of alcohol and contained detectable amounts of only one drug:
Fluvoxamine.
Harris had a therapeutic level of the drug in his bloodstream; his blood concentration of fluvoxamine was 390 ng/ml, which was right in the middle of the therapeutic range of 50-900 ng/ml (see below). This strongly indicated Harris was taking the SSRI at the time of the shootings (his accomplice Klebold evinced no detectable levels of any drug).
 Blood levels of fluvoxamine in Columbine shooter Eric Harris. For full autopsy results, visit: https://www.columbine-guide.com/columbine-autopsies-harris-klebold
Blood levels of fluvoxamine in Columbine shooter Eric Harris. For full autopsy results, visit: https://www.columbine-guide.com/columbine-autopsies-harris-klebold
When reports first surfaced that Harris was under a doctor's care and had been prescribed Luvox, the psychos from the psychiatric industry promptly went into damage control. American Psychiatric Association President Dr. Rodrigo Munoz said there was "no specific link between these drugs and violent behavior."
"Despite a decade of research, there is little valid evidence to prove a causal relationship between the use of anti-depressant medications and destructive behavior," Munoz said.
What nonsense.
As far back as 1994, the American Psychiatric Association's own Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) made it perfectly clear that antidepressants can cause mania. The link between antidepressants, mania and other mood disorders was mentioned repeatedly in the manual (see, for example, pages 329, 331, 332, 334, 336, 337, 351, 371, and 372). The DSM, it should be noted, is pretty much considered the bible of psychiatric diagnosis, to the point where it is cited not just in the medical and research world, but also legal cases.
In addition to its multiple references that antidepressants can cause mania, it also noted:
"... antisocial behaviors may accompany the Manic Episode. Ethical concerns may be disregarded even by those who are typically very conscientious ... The person may be hostile and physically threatening to others. Some individuals, especially those with psychotic features, may become physically assaultive or suicidal. Adverse consequences of a Manic Episode (e.g., involuntary hospitalization, difficulties with the law, or serious financial difficulties) often result from poor judgment and hyperactivity." (page 330).
By the time Munoz made his absurd remarks in 1999, fluvoxamine and other SSRIs had already established a solid track record for causing mania, hostility, aggression, violence and suicidal behaviour.
But never underestimate the capacity for denial in a profession effectively owned by Big Pharma.
And never underestimate the gullibility of the general population. Despite their penchant for inciting impulsivity, aggression and self-harm, SSRIs largely flew under the radar. The media was instead busy blaming Columbine on other convenient scapegoats – like Goth shock rocker Marilyn Manson. Seriously: With sensationalist headlines such as "Killers Worshipped Rock Freak Manson" and "Devil-Worshipping Maniac Told Kids To Kill", many came to believe Manson's music and imagery were the primary motivators behind the shootings. The subsequent investigation indicated Harris had little, if any interest in Manson, while Klebold was a casual Manson listener who seemed more interested in the German industrial bands Rammstein and KMFDM.[1]
While most people remained ignorant of the Columbine-SSRI connection and instead embraced all manner of strawmen, in 2002 Solvay quietly withdrew Luvox from the US market. The officially cited reason was "possible inaccuracies noted in the chemistry, manufacturing, and controls (CMC) section" of the new drug application filed by Solvay for fluvoxamine to the FDA. In 2007, Solvay Pharmaceuticals and Jazz Pharmaceuticals appealed to the FDA to have Luvox reinstated for sale in the US. This approval was granted, and Luvox returned to the US market in 2008.
Fluvoxamine: Remarkably Bad
To listen to the wanton bullpucky that flows from the medical profession and Big Pharma-sponsored researchers, fluvoxamine is a “remarkably safe” and effective drug.
The truth is that fluvoxamine is toxic junk of questionable efficacy.
As discussed in Part 1, administering anti-depressants to patients experiencing suicidal thoughts is an extremely precarious endeavour. Suicidal thoughts are common among those experiencing depression, and depression itself raises the risk of suicide. Thankfully, the majority of depressed people will not act upon their suicidal thoughts due to fear or lack of motivation. But when you place these patients on anti-depressants, most of which can exert stimulating effects, things can quickly take a turn for the worse. Giving a substance with stimulant properties to someone in a highly negative mind state can produce disastrous outcomes. In terms of violence and suicide, a formerly reluctant patient may now find himself in a highly charged, agitated, disinhibited and impulsive state. Where there was once a lack of motivation and willingness to act upon violent and suicidal thoughts, there now is ample.
“…the combination of agitation and depression adds up to an ‘agitated depression’. Agitated depression is one of the most dangerous conditions in psychiatry and is frequently the specific disorder associated with drug-induced violence and murder. The combination of fluvoxamine-induced agitation and depression, even without mania, could account for Eric Harris's disturbed mental state.”[Bold emphasis added]
Few people have studied the violent potential of antidepressant drugs as thoroughly as Dr Breggin. He has written several books on antidepressants and has served as a medical witness in numerous trials, including a suit brought against Solvay by one of the Columbine victims (that suit was ‘settled’ after the victim, Mark Taylor, was threatened with a counter suit and court costs by Solvay’s lawyers).
You’ll learn much more about Dr Breggin and his eye-opening findings in Part 3.
There were early warnings that fluvoxamine could have particularly stimulating effects.
A paper published way back in 1977 reported that electrical brain wave studies, conducted under double-blind conditions, "predicted that fluvoxamine will have psychotropic properties similar to those of predominantly 'stimulant' antidepressants."
And the warnings kept coming.
In 1993, Israeli researchers described three cases of fluvoxamine-induced mania. Because the participants were under close supervision, each case was identified quickly and the drug was reduced in dose or stopped. As a result, potentially violent outcomes were avoided. Had the patients been more secretive or the monitoring less effective, the results could have been disastrous – especially in the two male cases.
Case 1 was a 62-year old woman who, after 28 days on fluvoxamine, was “clearly expansive” (i.e. lacking restraint in the expression of her feelings), and her appearance was “extremely seductive.” The would-be senior seductress announced to clinic staff that she wanted to sell her house and divorce her husband. They promptly discontinued her fluvoxamine (and placed her back on thioridazine). On day 31, she “was hospitalized in a full-blown manic state, which included euphoric mood, grandiosity, loose association, and auditory hallucinations".
Case 2 was a 27-year-old man, first diagnosed at 19 with severe, recurrent, major depression. He was placed on fluvoxamine and the anti-psychotics perphenazine and biperiden, the latter two being dropped after his anxiety and psychotic symptoms subsided. However, he remained depressed, so his fluvoxamine dosage was increased to 300mg daily. A week later, "he became euphoric, displayed increased energy, and began cleaning the ward, singing, and kissing other patients. In addition, he was very irritable and anxious, exhibiting psychomotor agitation and describing fears that people were out to kill him." The fluvoxamine was stopped, and anti-psychotics were reinstated.
The third patient was a 48-year old male whose fluvoxamine dosage was gradually increased to 150 mg/d over a three-week period. The patient stated he no longer felt depressed, but during week 4 of fluvoxamine treatment, he experienced "motor hyperactivity; a decreased need for sleep; an increased ability to concentrate; and excessive social, sexual, and artistic prowess. Examination revealed an euphoric mood, grandiosity, logorrhea, and a very argumentative disposition."
When discussing these and five other patients who developed mania on fluvoxamine, the researchers noted "none of our patients developed manic behavior while being treated with other antidepressant drugs" (being the early 1990s, these other anti-depressants were all of the tricyclic variety – not SSRIs).
A 1997 report compared the safety and side effect profiles of four SSRIs: fluvoxamine (Luvox), fluoxetine (Prozac), paroxetine (Paxil), sertraline (Zoloft). The comparative data was obtained from four observational cohort studies in England, with a combined cohort exceeding 10,000 patients. The sex, age distributions and indications for prescribing the four SSRIs were very similar. As part of the study, questionnaires were posted to the prescribing doctors at least 6 months following the first prescription. Only 36% of the responding GPs reported fluvoxamine as effective, compared with approximately 60% for fluoxetine, sertraline and paroxetine. Fluvoxamine was associated with a higher incidence of adverse events than the other three SSRIs. Nausea/vomiting was the most frequently reported adverse clinical event in the first month of treatment for all four SSRIs, followed by malaise/lassitude, drowsiness/sedation, dizziness and headache/migraine.
Fluvoxamine use greatly increased the likelihood of these side effects, and also was much more likely to cause agitation and anxiety. "This study suggests that fluvoxamine compares unfavourably with fluoxetine, sertraline and paroxetine,” noted the study authors, “both in terms of reported effectiveness and the incidence of adverse events."
In a 2001 letter titled "Violent Acts Associated with Fluvoxamine", Japanese researchers described three cases of aggressive and violent behaviour induced by fluvoxamine. On fluvoxamine 150 mg/day, a 32-year-old woman became "irritable and aggressive, and she expressed impulsive violence during her disagreements with her husband and mother". She improved after her fluvoxamine dosage was reduced. This patient had previously used fluoxetine (Prozac), but had not experienced the kind of symptoms she suffered during fluvoxamine use.
A 29-year-old woman on 150 mg/day of fluvoxamine became nervous and irritable and then "impulsively violent" on fluvoxamine and was admitted to a psychiatric hospital. She improved with discontinuation of the drug and treatment with other medications.
A 28-year-old woman on 150 mg/day of fluvoxamine "exhibited signs of irritability and aggressive behavior, expressing violence toward her mother". She improved when her fluvoxamine was stopped and other medications instituted.
While all anti-depressants are suspect, the available evidence indicates fluvoxamine is a particularly problematic drug. The researchers concluded:
“Serotonergic abnormalities have been proposed as a neurobiological basis for aggression and impulsivity. The aggressive behaviour in these cases may be related to the fact that fluvoxamine is a more selective serotonin reuptake inhibitor than fluoxetine ... we wish to draw attention to the emergence of paradoxical effects such as impulsivity and aggressive behaviour induced by fluvoxamine treatment.”
Fluvoxamine and Suicide Risk
The two earliest papers I retrieved in my initial PubMed search for “fluvoxamine and suicide” were authored by Wagner and colleagues in 1992 and 1993:
Wagner W, et al. Review of Fluvoxamine Safety Database. Drugs, 1992; 43 (Suppl, 2): 48-54.
These two papers discuss a cohort of 24,624 subjects who participated in 54 worldwide marketing trials (i.e. trials performed after a drug has been released to market) of fluvoxamine. As an indication of the substandard quality of these trials, the overwhelming majority were uncontrolled studies (i.e. lacking randomization, blinding, placebo controls, etc.). “The studies reviewed were not planned, implemented, performed, or monitored according to consistent research guidelines,” reveal Wagner et al, “thus, the information on the case record forms was often incomplete, inconsistent, or even contradictory, and sometimes iIlegible or unreliable.”
Oh joy … nothing like crappy research to inspire confidence in a dubious drug that has been foisted upon an unsuspecting public!
The first thing to be noted about Wagner and his crew is that they worked for Solvay - the same company who brought us fluvoxamine. Little surprise then that, despite the substandard quality of their data, they wank on about how wonderful and safe the drug is.
Never mind that this dubious data shows a higher suicide rate for fluvoxamine than "active control" and placebo. To downplay this inconvenient fact, they emphasize how the small number of placebo and active control patients warrants caution in interpreting the results. That’s true, but the real question that begs to be asked is just what kind of appalling clinical research was Solvay doing when the placebo group had only … wait for it … 25 people?!?
I’m not kidding, it’s right there in Table 9 of the 1993 paper and Table X of the 1992 paper:
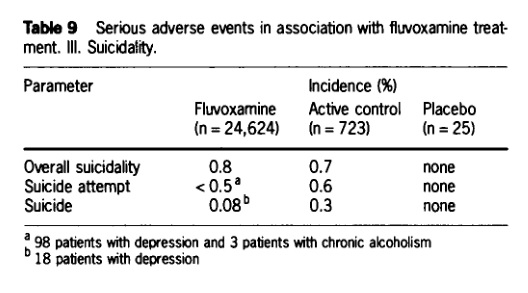 Over 24,000 drug subjects versus only 25 placebo subjects?
Over 24,000 drug subjects versus only 25 placebo subjects?
What a farce: When they said “uncontrolled”, they weren’t kidding!
Speaking of poorly conducted research, check out Table 4 from the 1992 and 1993 papers. The left column shows the duration of treatment in weeks, while the right column shows the number of research subjects who took fluvoxamine for the corresponding treatment duration.
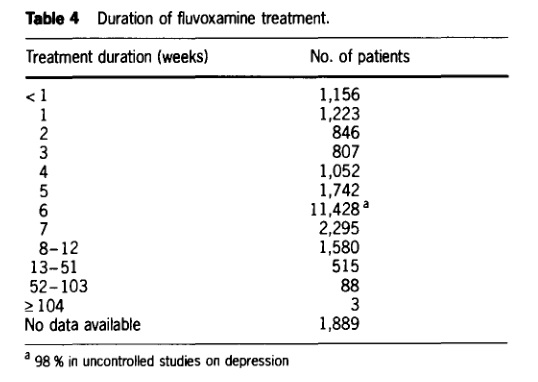
Excluding those falling into the “no data available” category, Table 4 lists a total of 22,735 subjects. A mere 19.7% of those treated with fluvoxamine were observed for longer than six weeks, and only a piddling 0.4% were followed for a year or more! By far the largest single observed duration of treatment was six weeks which, as I would later discover, is a typical duration for clinical trials of antidepressant drugs.
Stop and think about this for a moment: No-one really knows what these drugs do to the human brain, especially over the long-term. The clinical trials supposedly assessing the safety and efficacy of these drugs are typically measured in weeks, not years. And yet people are routinely prescribed these drugs for years on end!
The reality for people on antidepressants is that they are part of a massive experiment, a giant guessing game in which the only guaranteed winners are the profiteers who manufacture these drugs.
Although it is commonly prescribed for depression, the only FDA-approved uses for fluvoxamine are the treatment of social anxiety disorder and obsessive compulsive disorder (OCD). It is even prescribed to children and adolescents for the latter purpose, but judging by the Luvox package insert, the only premarketing study performed in this demographic involved a mere 57 children receiving fluvoxamine! In that ludicrously small ten-week trial, 2 out of the 57 pediatric patients (4%) treated with fluvoxamine experienced manic reactions, compared to none of 63 placebo patients. Again, the trial lasted a mere ten weeks, but this regrettable drug is routinely used for months and even years.
Regulatory agencies like the terminally corrupt FDA should be raked over hot coals for allowing this junk to be given to children and adolescents – an especially vulnerable group whose brains and nervous systems are still in the formative stage – based on such woefully inadequate research.
Swedish Swindlers
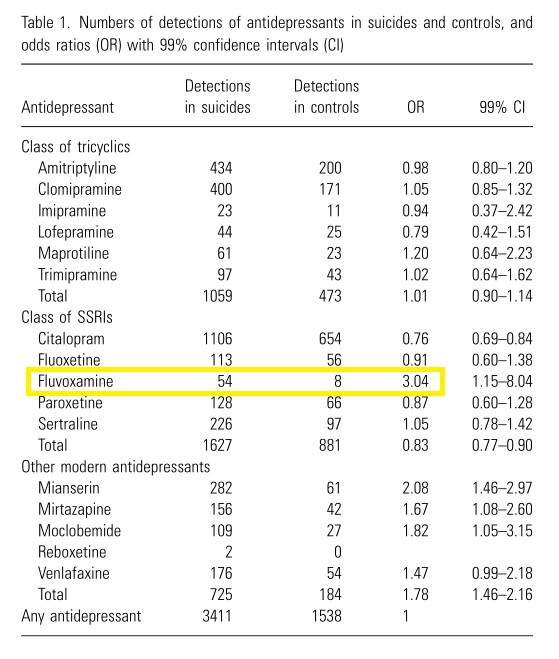
An especially revealing find from my initial search was a 2005 paper by Swedish researcher Göran Isacsson and his colleagues from the Karolinska Institutet in Stockholm. By the time we’re done discussing Isacsson and his ilk, you should begin to appreciate just how shady this whole antidepressant caper really is.
This wasn't a clinical trial or a collective review of such trials; it was an autopsy-based study. In Sweden, it is routine for unnatural deaths to be the subject of a forensic medical investigation, which includes a toxicological screening. About 200 substances are screened for, including all antidepressants.
During the years 1992–2000 in Sweden, there were 14,857 suicides (median age 49 years, 71% men). And so what Isacsson et al did was to compare the toxicology results of the 14,857 suicides with those in 26,422 deaths (median age 55 years, 73% men) by accident or natural causes during the same time period. Anti-depressants were detected 3,411 times in the suicide cases (23%), compared to 1,538 times in the non-suicide controls (5.8%).
Those figures show that suicide victims were around four times more likely to be using an anti-depressant than autopsied subjects who died of other causes. Of course, one would expect that most of those taking the anti-depressants were suffering mental health issues such as depression, which itself would have raised the risk of suicide. As such, it’s near impossible to tell just how much of that increased suicide risk stemmed from the subjects’ psychological states, and how much stemmed from anti-depressant use.
To circumvent this problem, Isacsson et al employed statistical sleight of hand and simply assigned the suicide and control groups the same frequency of anti-depressant use. That clearly is not what occurred in reality, and therefore makes this study completely useless for determining whether or not anti-depressants per se raised the risk of suicide.
What this study does still allow, however, is an insight into whether certain individual anti-depressants raised the risk of suicide in comparison to others. That’s because the odds ratio for each anti-depressant was ascertained by comparing its frequency of detection to the artificially contrived odds ratio of 1 for “Any antidepressant.”
Keeping this in mind, feast your eyes upon Table 1 from the Isacsson et al paper, and take a look at the highlighted data for fluvoxamine:
To the right hand side of the highlighted area, you can see an OR (odds ratio) figure of 3.04 for fluvoxamine. What this indicates is that, in comparison to users of other antidepressants, fluvoxamine users were 3 times as likely to commit suicide!
To any rational human being, such a huge risk increase would be a major cause for concern, but Isacsson et al weren’t at all fussed. Instead of sounding caution, they instead attempted to rationalize away the disturbing fluvoxamine finding with the following piece of gobbledegook:
"The high OR for fluvoxamine is inconsistent with other data(11) and maybe explained by the fact that it was the first SSRI introduced in Sweden and it was seldom used after 1994. In 1990–94, SSRIs were used less than [tricyclic antidepressants], but often in TCA-refractory cases and when a suicide risk was suspected (12), so that all SSRIs had higher risks during that period (8,13)"
If that passage sounds to you like a load of convoluted, apologist bullshit, rest assured - it is.
First of all, a higher suicide risk for fluvoxamine is not inconsistent with other data. Other data does indeed show a higher risk, but instead of citing this accessible research, Isaacson and his co-workers instead reference a hard-to-access book that will set you back US $115.00 (Selective 5-Ht Reuptake Inhibitors: Novel or Commonplace Agents? Basel, Karger, 1988). I tried searching for this title at the medical libraries I have access to, with no luck.
When someone ignores readily accessible research and instead cites a book that most people cannot get access to and cannot afford, I immediately smell a big, stinking pile of caca de vaca.
We’ll find out more about Isacsson and his dubious motivations in a moment, but first let’s look at some of the other claims he and his colleagues made in the above passage. For us to take seriously the claim that fluvoxamine only showed an elevated risk prior to 1994, and that this was due solely to the patient group it was prescribed to in that period, we'd need to know the dates of death for the autopsied victims who showed elevated blood levels of fluvoxamine, and their clinical history.
Of course, this information is not supplied.
However, reference 13 in the Isacsson 2005 paper (Isaacson 1997) does contain suicide data for fluvoxamine and other SSRIs during the period 1992-1994. Before we examine this data, it behoves me to address a statement made in the introduction of Isacsson 1997:
"The huge increase in the use of antidepressants in Sweden since 1990-1991 has been paralleled by a significant decrease in suicide rates."
There’s no mistaking the intent of this statement: It is designed to give the reader the distinct impression that a drop in Swedish suicide rates was caused by the introduction of SSRIs.
Which is absolute rubbish.
As Reseland et al explained in a 2006 paper, the suicide rate in Sweden began going down before SSRI drugs were introduced. Whether it was economic improvements, or changes in social factors, or whatever - SSRIs do not even begin to explain the downturn in suicide rates. In more recent years, Sweden's suicide rate has pretty much flat-lined, despite it being among the world's most voracious consumers of anti-depressants.
Inspired by the shady claims of Iasacsson et al, Swedish journalist Jan Larsson investigated this very issue, focusing her investigation on suicides in young women. She published her findings in a 2017 paper.
She found that suicides among young women declined from the 1980s, but then reversed trajectory around the turn of the millennium. During the period 1999–2013, suicide rates among young Swedish women increased.
During that same period, the prescription of antidepressants and other psychotropic drugs for young women rose sharply.
Larsson also discovered that in the forensic toxicological screening carried out on women who committed suicide there was a sharp rise in detection of antidepressants and other psychotropic drugs. Data from these analyses for young women showed traces of antidepressants were found in 13% of cases for 1999 and 41% of cases for 2013.
Antidepressants reduce suicide risk?
Il mio culo…
As Larsson concluded:
"An increasingly larger proportion of young women who later committed suicide, had in the last few years been treated with antidepressants, prior to and at the time of the suicide. The previous assumptions that treatment with antidepressants would lead to a drastic reduction in suicide rates, are incorrect for the population of young women. On the contrary, it was found that an increasing tendency of completed suicides follow the increased prescription of antidepressants."
Remember how Isacsson et al claimed that the whopping 3.04 odds ratio for fluvoxamine was just an unlucky anomaly resulting from unfavourable pre-1994 circumstances? When we bypass their clearly biased verbiage and go straight to Table 2 in their 1997 paper, we learn that, in the period 1992-1994, fluvoxamine use was associated with a standard mortality ratio of 1.6. Meaning, fluvoxamine was 1.6 times more likely to show up in the blood of autopsied suicide victims than amitriptyline, which at the time was the most widely prescribed antidepressant in Sweden and hence served as the reference drug. Another SSRI, citalopram, evinced a SMR of 1.76.
Given that fluvoxamine was associated with far greater odds of suicide when including data post-1994, this makes Isacsson’s pre-1994 pro-fluvoxamine rationalizations look especially flimsy.
There’s a big difference between the 60% greater SMR evinced by fluvoxamine when compared to amitriptyline, and the 300% greater odds ratio seen when fluvoxamine was compared to all other antidepressants, so the excess risk incorporated into the latter figure either occurred before 1992, or after 1994.
In a 1994 BMJ article, Isacsson et al examined blood levels of antidepressants in autopsied suicide victims for the period 1991-1992, but separate figures for fluvoxamine were not included in their analysis. But the authors did let slip in that paper that SSRI drugs "were introduced into Sweden during 1990-1 and then had a minimal share of the market."
So how on Earth Isacsson et al can proclaim the alarming 3.04 odds ratio imparted by fluvoxamine is simply an artefact of unfavourable pre-1994 circumstances remains a complete mystery.
In response to the 60% risk increase between 1992-1994, Isacsson et al admitted in their 1997 paper:
"When related to their use, significant over-risks compared to amitriptyline were found for trimipramine, nortriptyline, citalopram, fluvoxamine, moclobemide and mianserin."[Bold emphasis added]
The researchers again tried to downplay this worrying "over-risk" by claiming:
"Most people committing suicide were not taking antidepressants immediately before their death, even though 40-85% may have been depressed. Undertreatment and therapeutic failure are the main problems with antidepressants, not the risk of using antidepressants in overdose."
Fuck me.
Whether or not most suicide victims were taking antidepressants "immediately before their death", the fact remains certain antidepressants were detected with disportionate frequency among those who had taken their own lives.
As for their genius statement that "Undertreatment and therapeutic failure are the main problems with antidepressants" ...
Fuck. Me. Again.
Their response to data showing antidepressants increase suicide risk is to whine about “undertreatment”?!
What … not enough people are dying??
As for “therapeutic failure are the main problems with antidepressants” … no shit! I’d say people killing themselves at an increased rate while on drugs that are heavily promoted as mood-enhancers is a therapeutic failure of epic proportions and a major problem!
What’s Your Real Agenda, Göran?
As I read the idiotic rationalizations put forward by Isacsson and his buddies, a question kept burning in my mind:
Why were they trying so hard to downplay the increased suicide risk of fluvoxamine and other antidepressants?
Their data clearly showed a markedly higher risk for fluvoxamine, yet they did all they could to brush it off as some kind of inconsequential anomaly.
In an attempt to discover why they would behave in such a reckless manner, I started digging around online to find out more about Isacsson. It didn’t take me long to stumble upon this:
http://psychrights.org/2013/130103JanneLarssonAntidepressantDataDestroyed.pdf
Lawdy, lawdy.
It seems Isacsson is staunchly committed to downplaying the suicide risk of anti-depressants, and he’s not above misrepresenting research data in order to achieve this end.
“Unintentionally”, of course [COUGH, COUGH].
As Larsson explains, in 2010 Isacsson published an article titled “Antidepressant medication prevents suicide in depression” in the journal Acta Psychiatrica Scandinavica, but the article was subsequently retracted in March 2012. The reason given for the retraction was “unintentional errors in the analysis of the data presented”.
Isacsson's 2010 article was built upon his alleged finding that “only” 15.2% of 1,077 patients admitted for psychiatric care for depression had measurable amounts of antidepressants in their blood at the time of suicide. Isacsson compared this figure with the percentage of antidepressants in suicides in other patient groups, which was around 35%. He then concluded:
“The finding that in-patient care for depression did not increase the probability of the detection of antidepressants in suicides is difficult to explain other than by the assumption that a substantial number of depressed individuals were saved from suicide by postdischarge treatment with antidepressant medication.”
The article was filled with Isacsson's trademark fanciful speculations and assumptions, and as in all his other papers he concluded antidepressants protected against suicide – despite everything that has emerged in clinical trials about these drugs causing suicidal behaviour.
According to Larsson, Isacsson’s 2010 assertions were widely published in Swedish newspapers, with headlines like “Antidepressants prevent suicide.”
After Isacsson's paper was retracted, and detecting the unmistakable stench of bullshit, Larsson made an FOI request to Karolinska Institutet in June 2012 to get the corrected figures. She specifically sought the document containing the correct percentage of antidepressants for those “who committed suicide and who had previously been treated at a psychiatric clinic for depression” (the aforementioned group of 1,077 persons).
The answer from Karolinska Institutet: This is confidential information, no data can be released.
What followed was a five month legal process to get access to the data. The whole time, Karolinska Institutet claimed all the data in this research project were confidential. After being compelled by the court, Karolinska Institutet finally claimed the correct figures did not exist at the time of the FOI-request, but that the correct figures now had been produced.
Wait a minute: First the Institutet claimed the data were confidential, now they were claiming they had only just been produced?!?
The correct data was of course produced before, and was the very reason the original article had to be retracted.
Even more alarming than Karolinska Institutet’s duplicity was the degree to which Isacsson had misrepresented the suicide risk. The Karolinska Institutet admitted to the court:
"The result shows that ‘the correct percentage’ is 56, meaning that of the persons who had been treated for depression in psychiatric care in the last five years before suicide, 56% had antidepressants in their blood when they committed suicide.”
So finally it was revealed that the 15.2% was in fact 56% – an increase of 268% (from 164 persons to 603)!
This disgraceful episode was yet another example of the shamelessly corrupt behaviour that has come to characterize Big Pharma and the researchers and universities on its payroll.
Fluvoxamine and Violent Suicide
A study published in 2005, headed by Harvard Medical School's Sebastian Schneeweiss, compared the suicide risk of various antidepressants among a cohort of 287,543 Canadian adults. The drug that served as the de facto standard to which all others were compared was flouxetine. After extensive statistical adjustment for numerous potential confounders, the researchers reported "no clinically meaningful variation in the risk of suicide and suicide attempt between antidepressant agents" (including fluvoxamine) when compared with fluoxetine. However, scrolling all the way past the study text and examining the actual tabulated data shows that, even after extensive statistical adjustment, fluvoxamine exhibited a greater association with “Violent Composite Outcomes”. This included violent suicide attempts and violent completed suicides, defined as follows: hanging, gunshot or explosion, jumping or lying in front of a moving object, vehicle collision, electrocution, and self-immolation. This is noteworthy because there is research to show that not only does SSRI use increase suicide risk, but it increases the likelihood of using violent means (e.g. gunshot/hanging/setting fire to one's self) as opposed to more 'gentle' methods (e.g. drug overdose) during suicide attempts.
Fluvoxamine Sucks – as Do the Other SSRIs.
Clearly, fluvoxamine is a highly suspect drug. The available research does not even begin to support the oft-repeated claim that is a “remarkably safe” and “highly effective” drug.
At this point, some readers may be thinking, “well then, I’ll just use a different SSRI if the need ever arises.” Before embracing this line of thought, I would strongly urge such readers to stay tuned for Part 3, where I’ll present the less-than-stellar track record of the remaining SSRIs.
Ciao,
Anthony.
---
Notes:
Section on initial SSRI availability updated 12 May 2019.
The following Columbine information added 15 December 2019:
1. In an FBI-recorded telephone conversation, two of Harris' friends expressed their dismay at the media sensationalism. “[T]he media’s just full of shit you know,” lamented Philip Duran. Harris was more interested in “Hitler stuff” than Manson, noted Christopher Morris, while Klebold was at best a casual Manson listener. See pages JC-001-010826 to JC-001-010827 at https://schoolshooters.info/sites/default/files/JCSO%20Pages%2010%2C001%20-%2010%2C937.pdf
When law enforcement searched the Klebold residence, they found a single Marilyn Manson CD. Search records for the Harris residence show no mention of CDs by Manson or any other artists. See pages JC-001-025730 and JC-001-025592 to JC-001-02595 at https://schoolshooters.info/sites/default/files/JCSO_25658_to_25889.pdf
When interviewed by law enforcement officials, Mrs Klebold said Dylan’s room had a poster each of Marilyn Manson and the Nine Inch Nails, another band from the “industrial” genre. The Klebold’s also indicated their son listened to techno music, and particularly liked the German bands Rammstein and KMFDM. When Mrs Klebold asked Dylan about the Manson poster, “he told her it that it didn’t mean anything and that he didn’t really listen to lyrics of Marilyn Manson music, however, did listen to the music." While Dylan evidently had an affinity for the hard-edged industrial sound, there was nothing to suggest a preoccupation with Manson or his lyrical content. See page JC-001-010511 at https://schoolshooters.info/sites/default/files/JCSO%20Pages%2010%2C001%20-%2010%2C937.pdf
---
Anthony Colpo is an independent researcher, physical conditioning specialist, and author of the groundbreaking books The Fat Loss Bible, The Great Cholesterol Con and Whole Grains, Empty Promises.
For more information on Anthony's books, click here.
---
The Mandatory “I Ain’t Your Mama, So Think For Yourself and Take Responsibility for Your Own Actions” Disclaimer: All content on this web site is provided for information and education purposes only. Individuals wishing to make changes to their dietary, lifestyle, exercise or medication regimens should do so in conjunction with a competent, knowledgeable and empathetic medical professional. Anyone who chooses to apply the information on this web site does so of their own volition and their own risk. The owner and contributors to this site accept no responsibility or liability whatsoever for any harm, real or imagined, from the use or dissemination of information contained on this site. If these conditions are not agreeable to the reader, he/she is advised to leave this site immediately.