The COVID-19 vaccine story is currently dominated by three drugs: The Moderna and Pfizer-BioNTech mRNA drugs, and the Oxford-AstraZeneca vaccine. I recently dissected the dubious science behind the latter two drugs here and here.
As my previous articles explained at length, the science supposedly supporting the Pfizer-BioNTech and Oxford-AstraZeneca drugs is a bad joke. It is also a matter of record that both Pfizer and AstraZeneca are corporate criminals, having racked up billions in penalties over the last two decades for a host of dishonesty offenses including fraud, bribery and false marketing.
The world is now being asked to forgive and forget the serial criminal dishonesty of these two pharma giants, and allow themselves to be injected with their poorly tested vaccines.
But what about Moderna? While most people know of Pfizer and AstraZeneca, many knew nothing of Moderna until it seemingly popped out of nowhere to become one of the main players in the COVID vaccine race.
In this article, I'll detail the questionable past of the young company. I'll also explain how, despite Moderna's poor track record, its COVID vaccine candidate became the first to advance to the initial phase of federally-funded clinical study. In the next article, we'll delve into the science - or lack thereof - behind its highly questionable mRNA COVID-19 vaccine.
The Questionable Past of Moderna
The mRNA COVID-19 'vaccines' from Moderna and Pfizer-BioNTech are not really vaccines, at least not in the traditional sense. Instead, they are gene-modifying agents that insert synthetic messenger RNA into your cells. This synthetic mRNA contains instructions for your cells to produce a protein resembling the spike protein found on the exterior surface of a COVID-19 molecule. Your cells then eject this protein. Once outside the cells, it triggers the production of COVID-19 antibodies.
These antibodies will supposedly protect you against the 'deadly' COVID-19 which, as repeated studies have shown, is not very deadly at all. Even the CDC acknowledges the very low infection fatality rate (IFR) of COVID-19. Its best guess estimates show the only group where the IFR exceeds 0.5% is in the over 70s. In every other group, the IFR becomes a non-event, especially when compared with far more serious threats like cardiovascular disease, cancer, traffic accidents, drug and alcohol abuse, and suicide.
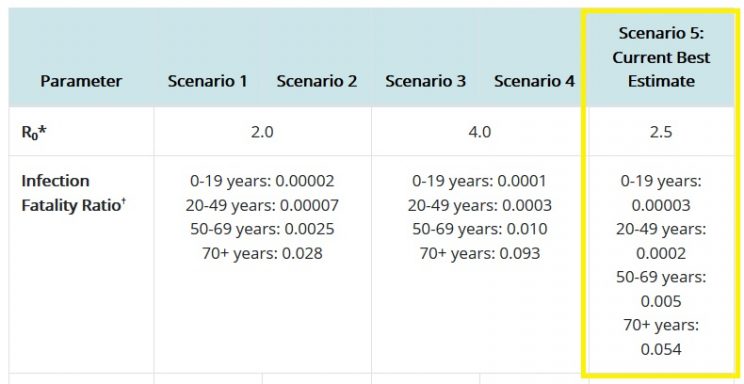 Source: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html
Source: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html
So ... if you are going to unleash a drug on the world for a not-very-lethal virus, and insist everyone take it, then you better damn well make sure that drug is safer than home-baked apple pie.
But there is nothing sweet and wholesome about the Moderna story, nor the vaccine that has emerged from it.
Moderna: Big on Hype, Short on Results
The early pioneer of mRNA technology was Hungarian-born scientist Katalin Karikó, who is now Senior Vice President at BioNTech. Driven by a belief in the revolutionary potential of mRNA technology to treat a host of diseases, she persevered with mRNA research despite a continual string of disappointing results. The enduring monkey wrench was that injecting synthetic mRNA routinely caused an unwanted immune response; the body sensed a foreign intruder and went into attack mode. The culprit was narrowed to one of the altered, synthetic RNA nucleosides which seemed to be triggering the immune system. Karikó and her Penn State colleague Drew Weissman swapped it out for a slightly modified version, creating a hybrid mRNA that could seemingly sneak its way into cells without alerting the body’s defenses. Well, at least under their experimental lab conditions.
This discovery was first published in 2005 and while it generally raised little fanfare, it did catch the attention of one Derrick Rossi at the Harvard Stem Cell Institute and Harvard Medical School. Rossi and his team experimented with the technology in his Harvard lab, and the researcher concluded he was on to something big. But with no entreprenurial experience, he needed advice. So he turned to Harvard colleague Tim Springer, who had been successful in taking his discoveries to market.
This led to an appointment with Bob Langer, a prolific MIT scientist and entrepreneur. Langer liked what he heard. So too did Noubar Afeyan, who the duo approached for funding. Afeyan was the CEO of Flagship Ventures, a venture capital firm investing in biotech start-ups.
The next big name on board was Ken Chien, a physician-researcher at Harvard and Sweden's Karolinska Institute, known for his work on heart stem cells. For years, Chien had been trying to figure out how to effectively regenerate heart muscle and vessels damaged by heart attack, and Rossi’s technology offered him a potential solution. He wanted in.
Rossi, Langer, Afeyan, and Chien banded together and in September 2010 announced the formation of Moderna. The following year, Stephane Bancel was lured from French diagnostics company BioMérieux to become Moderna's first CEO.
It's also worth mentioning that in 2017, a certain Moncef Slaoui joined Moderna's board of directors, after leaving his job at GlaxoSmithKline. Prior to joining the Moderna board, Slaoui had worked at GSK for some thirty years, starting as a vaccine researcher before being appointed head of research and development. Slaoui's name will resurface later in this story, under circumstances that hardly inspire confidence in the impartiality and independence of government health agencies.
The hyperbole from the new company came thick and fast, and investors piled in, hoping to catch a ride aboard the next Genentech. “We’re going to rewrite the book of biotech on this company," claimed the exuberant Bancel. "Everything’s going to be different. We’ll do nothing by the playbook.”
“It’s the perfect personalized medicine,” Bancel added. “You make your own drug.”
But the research results never lived up to the outlandish PR. When journalist Catherine Elton watched a Moderna scientist 'successfully' toy with modified mRNA in a lab experiment, Moderna’s chief scientific officer, Tony de Fougerolles, patted the scientist on the back, then told Elton, “In five years, that mRNA you just saw there might treat a disease that is currently not druggable.”
That was back in 2013.
Five years later, Moderna was still desperately trying to produce a viable mRNA drug. In fact, until the rushed "emergency authorization" of Moderna's mRNA COVID drug in December 2020, the company was nowhere close to successfully developing a single, approvable drug.
It wasn't for lack of trying.
By early 2013, Moderna had already filed 90 patent applications containing more than 4,500 claims.
In March 2013, Moderna and AstraZeneca signed a five-year agreement to discover, develop, and commercialize mRNA therapies for cardiovascular, metabolic, and kidney diseases, and even cancer. The agreement included a $240 million upfront payment to Moderna, a payment that was "one of the largest ever initial payments in a pharmaceutical industry licensing deal that does not involve a drug already being tested in clinical trials", and an 8% share in Moderna.
As of April 2020, only one candidate from the Moderna-AstraZeneca collaboration had even passed Phase I trials, the earliest stage of human research for drugs hoping to one day make it to market. The drug was a treatment for myocardial ischemia, labelled AZD8601.
In fact, right before winning federal funding for COVID vaccine development in March 2020, only 3 of Moderna's projects had made it past Phase 1 testing, and only one of these was a vaccine (a cytomegalovirus vaccine codenamed mRNA-1647).
In January 2014, Moderna and Alexion Pharmaceuticals entered a $125 million to develop rare orphan disease treatments, using Moderna's mRNA technology. However, by 2016, the program had been scrapped as animal trials showed Moderna's treatment would never be safe enough for use in humans.
In February 2016, an op-ed in Nature criticized Moderna for not publishing any peer-reviewed papers on its technology, and compared its secretive approach to that of the controversially failed Theranos.
In September 2018, Thrillist published an article titled, "Why This Secretive Tech Start-Up Could Be The Next Theranos", criticizing Moderna's secrecy and lack of scientific validation of its research. Noting the parallels with Theranos, Thrillist said Moderna "keeps investors in the dark," having "published no data supporting its vaunted technology ... Outside venture capitalists said Moderna has so many investors clamoring to get in that it can afford to turn away any who ask too many questions. Some small players have been given only a peek at Moderna's data before committing millions to the company."
Like Elizabeth Holmes, the eccentric CEO of the scandalized Theranos, Bancel is not a scientist. Said Thrillist, "his qualifications to make major scientific decisions at Moderna are also questionable ... Bancel had no experience running a drug development operation ... he'd spent most of his career in sales and operations, not science ... He is listed as a co-inventor on more than 100 of Moderna's early patent applications, unusual for a CEO who is not a PhD scientist."
Thrillist also succinctly summed up the problems with Moderna's supposedly world-changing technology. "The mRNA treatments it's trying to develop are potentially very dangerous ... Theoretically, the revolutionary treatments could target and reprogram specific cells, allowing our own bodies to cure themselves of disease with repeated doses over time."
But, as Thrillist noted, "A number of Big Pharma companies have attempted to develop similar technologies, but abandoned them over fear that they produced prohibitively dangerous patient side effects. Delivery - actually getting RNA into cells - has long bedeviled the whole field. On their own, RNA molecules have a hard time reaching their targets. They work better if they're wrapped up in a delivery mechanism, such as nanoparticles made of lipids. But those nanoparticles can lead to dangerous side effects, especially if a patient has to take repeated doses over months or years."
Indeed, severe allergy-like reactions occurred in at least eight people who received the Pfizer-BioNTech vaccine during a 2-week period after the vaccine rollout in December 2020. Like the Moderna vaccine, the Pfizer-BioNTech coats its synthetic mRNA with liposomes and a chemical called polyethylene glycol (PEG). PEG has never been used before in an approved vaccine, but is found in many drugs that have triggered anaphylaxis — a potentially life-threatening reaction that can cause rashes, plummeting blood pressure, shortness of breath, and rapid heartbeat. Fauci's NIH National Institute of Allergy and Infectious Diseases (NIAID) scrambled to convene several meetings to discuss the allergic reactions with representatives of Pfizer and Moderna, independent scientists and physicians, and the FDA. Little has since been said about the problem, even though adverse event reports from the vaccines continue to mount at a worrying rate. Instead, the public is being fed the brazen lie that the mRNA vaccines have been thoroughly tested and proven Safe and Effective!™
Moderna's Toxic Work Culture and High Staff Turnover
A 2016 STAT investigation found the company’s "caustic work environment has for years driven away top talent and that behind its obsession with secrecy, there are signs Moderna has run into roadblocks with its most ambitious projects." STAT interviews with more than twenty current and former employees and associates "suggest Bancel has hampered progress at Moderna because of his ego, his need to assert control and his impatience with the setbacks that are an inevitable part of science."
Former employees said they felt the business-minded Bancel prized the company’s ever-increasing stock market valuation over its science. As he pursued a "complex and risky" drug development strategy, Bancel built a culture of recrimination at Moderna, they said. Failed experiments were met with reprimands and even on-the-spot firings. They recalled abusive emails, dressings down at company meetings, exceedingly long hours, and unexplained terminations.
Others didn't wait around to suffer the full weight of Bancel's wrath. At least a dozen highly placed executives had quit in the previous four years, including heads of finance, technology, manufacturing, and science. In the twelve months prior to the STAT story, two respected scientists leading Moderna’s cancer and rare disease programs resigned, even though the company’s remarkable fundraising success had put ample resources at their disposal. Each had been at the company less than 18 months.
Even co-founder Rossi, whose vision was the initial seed for Moderna's formation, headed for the exit in 2014 as a result of internal squabbling.
Lower-ranking employees, meanwhile, said they were disappointed and confused by Moderna’s pivot to less ambitious — and less transformative — treatments. Despite boasting of world-changing technology that would one day cure the likes of cancer and heart disease, Moderna had switched its focus to vaccines.
While this change of course may have perplexed lower-ranking employees, there was actually a simple explanation.
Behind the scenes, the company’s scientists were running into a familiar problem. In animal studies, the ideal dose of their leading mRNA drug was triggering dangerous immune reactions — the kind for which Karikó and Weissman had supposedly improvised a workaround. A lower dose had proved too weak to show any benefits. If repeated doses of mRNA were too toxic to test in human beings, the company would have to rely on something requiring only one or two injections to show an effect. And so Moderna put its stable of experimental drugs on the backburner and morphed into a vaccines company.
Money Talks. Sadly, So Does Bullshit
Moderna sounds more reminiscent of Enron than the next Genentech. But while the company was repeatedly coming up short in the quest to make a viable mRNA drug, it sure knew how to whip investors into a frenzy with its lavish promises of world-changing therapies. When Moderna went public in December 2018, it was the largest biotech initial public offering in history, raising over $600 million on NASDAQ, and implying an overall valuation of $7.5 billion for the entire company.
As of this writing, the company currently enjoys a market capitalization of $71 billion. As some of the more grounded market analysts have noted, this absurd valuation is not justified by the underlying fundamentals. The US is unquestionably Moderna's biggest market. But even if the company sold the two-shot vaccine to every single American at the government-agreed price of $15 per dose, this would only equate to $9.2 billion in sales (not profit). For a company with no other presently viable drugs, a $71 billion market cap is insane.
Which might help explain the massive insider selling that has been taking place at Moderna. The company's CEOs have been dumping Moderna shares like bad smells at a whole-grain eating contest.
So the question is, how did an overhyped, overvalued company with absolutely no track record of successful drug production achieve something many older, established companies failed to do? Namely, become one of the very first companies to win "emergency use authorization" for a COVID-19 'vaccine'?
With government help and taxpayer money, of course.
The White House Version of 'Shark Tank'
One of the most oft-repeated criticisms leveled at former President Trump is his "mishandling" of the COVID-19 'crisis.'
Trump did indeed screw the pooch with his response to COVID-19, but not in the way sufferers of Trump Derangement Syndrome would have you believe. Right from the word go, everything about the official COVID story reeked of unbridled bullshit. We were told the virus emanated from a wet market in Wuhan, China, even though many of the initial patients - including the the first identified case - had no link to the market. We were told the virus 'jumped' to humans by a mythical feat of 'zoonotic transfer' after someone allegedly ate an infected bat ... no wait, it might have been a pangolin ... hang on, maybe it was a snake ... purchased from the market.
While there was never a shred of evidence to support this nonsensical claim, it is an established fact that a NIH-funded BSL-4 lab in Wuhan was deliberately creating virulent hybrid strains of bat coronaviruses. However, those who pointed out this inconvenient fact were quickly shouted down as "conspiracists," while the ridiculous wet market tale was embraced as the official mainstream origin story.
Then there was the tsunami of hysteria portraying the virus as humankind's Day of Reckoning. The virus was so contagious and so deadly, we were told, it mandated a new anti-social world order of physical ('social') distancing, mask-wearing and inhumane lockdowns. All the while, study after study showed that the virus had an infection fatality rate that could be measured in fractions of a percent.
To scare people into submission as a result of this otherwise weak virus, the complicit media needed a rapidly-increasing death count. An especially brazen and open admission the whole thing was an overblown sham came at a 7 April 2020 Whitehouse press briefing. After Trump handed the microphone over to Coronavirus Task Force member Joanne Birx, she nonchalantly admitted the US had taken a very "liberal" approach to counting COVID deaths. So liberal, in fact, that any death accompanied by a positive COVID result would be listed as a COVID death - even when the real cause of death was heart attack or kidney failure!
When a journalist queried this approach, Birx fumbled, leading Fauci to take the mike and confirm the alarming revelation that had just spilled from Birx mouth. This should have bought the whole charade to a crashing halt, as it confirmed the official COVID death count was a blatant fraud. The press of course, has long since proven it is a propaganda arm of the global elite, rather than a vector of factual information. But Trump was sitting there and, unless he was daydreaming about golf or reminiscing about one of his more memorable Miss Universe conquests, should have heard what Birx and Fauci just admitted to.
This is where Trump really screwed up on COVID-19. Instead of decisively calling bullshit on what was clearly a sham perpetuated by the same class of Deep State, swamp-dwelling malevolents he promised to chase out of town, he ended up buying into it. He should have immediately fired pseudoscientists like Birx and Fauci from his Coronavirus Task Force, and instead appointed scientists who promised not to count fatal heart attacks and kidney failure as COVID deaths. Instead, he let them remain. Not only did this ensure the perpetuation of a fraudulent COVID death count, where authorities even felt uninhibited about counting murder-suicides by firearm as COVID deaths, it guaranteed the brazen BS and fear-generation campaign would continue unabated.
Granted, Trump may not have embraced the COVID sham with the vigor of his clearly sociopathic Democrat opponents - he did ignore calls to keep US borders closed to travellers, and when he himself contracted COVID-19, the 74 year old quickly bounced back and told Americans, “Don’t let it dominate you. Don’t let it take over your lives.”
But at some point, Trump fully embraced the vaccine charade. Not only did he embrace it, he believed it to be of such critical importance he established the farce that was "Operation Warp Speed." This truly warped endeavour was based on the reality-defying premise that the usual drug development process - and the many years required to properly establish safety and efficacy - could somehow magically be condensed into a few months.
On 2 March 2020, the White House held a roundtable convened by Trump aimed at speeding the development of a COVID vaccine. As CNN reporters noted, "it almost felt like an episode of 'Shark Tank.'"
One by one, vaccine developers pitched their product. John Shiver, the head of R&D for Sanofi Pasteur vaccines said he could have a product ready for the clinic in a year - perhaps a vaccine for the public in as "few as several years."
Trump seemed unmoved. "Right. OK. Thank you very much," he said.
Next was Lenny Schleifer, the founder and CEO of Regeneron, who talked of producing 200,000 vaccine doses per month from his factory, starting in August, "if all goes well."
This got Trump's attention. He leaned over the table and interrupted mid-pitch. "So that process would be faster than John's?" he asked, pointing at Shiver.
"It would be," Schleifer said, adding that the process could take "weeks to months."
The next CEO was Bancel. He glanced across the table at Fauci, and made a point of mentioning he was "very proud to [already] be working with the US government" and "Dr. Fauci's team at the NIH." Bancel went on to say he needed just "a few months" to start Phase Two of the three-phase clinical process that often takes more than a decade.
Trump, noted CNN, seemed to tune out everything but the talk of time.
"So you're talking over the next few months, you think you could have a vaccine?" he asked.
"Correct. Correct," Bancel said, then added, "with phase two," after he noted Fauci shifting in his chair across the table. Fauci then interjected for Trump's benefit: "You won't have a vaccine. You'll have a vaccine to go into testing."
 The 2 March 2020 White House roundtable meeting, which saw Moderna become the first federally-funded COVID vaccine developer.
The 2 March 2020 White House roundtable meeting, which saw Moderna become the first federally-funded COVID vaccine developer.
The very next day, the FDA green-lit Moderna's drug for trial, making it the first COVID vaccine candidate to advance to the first phase of federally-funded clinical study.
Again, this was quite a feat, considering Moderna had never brought a product to market, or gotten any of its nine or so vaccine candidates approved for use by the FDA. It had also never brought a product to the third and final phase of a clinical trial.
But Trump and the Whitehouse weren't finished. On 15 May 2020, they officially announced Operation Warp Speed, which had a war chest of $10 billion of taxpayer money on offer for promising vaccine candidates. The figure was later increased to $18 billion in October 2020.
To top it all off, the person appointed to head Operation Warp Speed was none other than Moderna board member, Moncef Slaoui.
With Operation Warp Speed, Trump did three very regrettable things. He lent credibility to the false claim COVID-19 was so deadly it required a vaccine equivalent of the Manhattan Project. He also established a very dangerous precedent, in which any overhyped virus can now be used to justify "emergency use authorization," conveniently allowing new and poorly tested drugs to be rushed to market on false pretenses. And by appointing someone who came straight from a vaccine company to lead the whole charade, Trump signaled that blatant conflicts of interest were A-OK in this already regrettable escapade.
To anyone with the requisite intelligence and powers of reason, this is what will go down as Trump's greatest failure - along with his pandering to the heinous Saudi regime and his indifference to the plight of Julian Assange ("I love WikiLeaks," he told listeners during his 2016 campaign).
Interestingly, his overly cosy relationship with the Saudis - which involved turning a blind eye to their murder of a journalist and the horrors in Yemeni - attracted relatively little criticism from Democrats or the mainstream press, as did his terribly misguided Operation Warp Speed. I guess both sides of the political divide understand war and arms sales are good for the economy, and that giving drug companies something to exuberantly hype is good for the stockmarket. As for Assange, no-one in authority likes a pesky whistle-blower, do they?
So that's Moderna: An overpromising, underdelivering company that became the first company to get US federal backing for COVID vaccine development, not because of its successful track record (it does not have one) but because its quick-talking CEO was able to tell Trump what he wanted to hear.
In the next post, I'll pull apart the Moderna trial results of its mRNA 'vaccine' and put them up against the drug's accumulating adverse event reports.
Stay tuned.
If You Found This Article Helpful, Please Consider Leaving a Tip
This site is self-funded and relies on reader generosity. Researching and writing articles like this takes a lot of time, so any and all tips are greatly appreciated!
